21+ how to calculate qsp
Plug in given concentration values. Web The key difference between Ksp and Qsp is that Ksp indicates the solubility of a substance whereas Qsp indicates the current state of a solution.
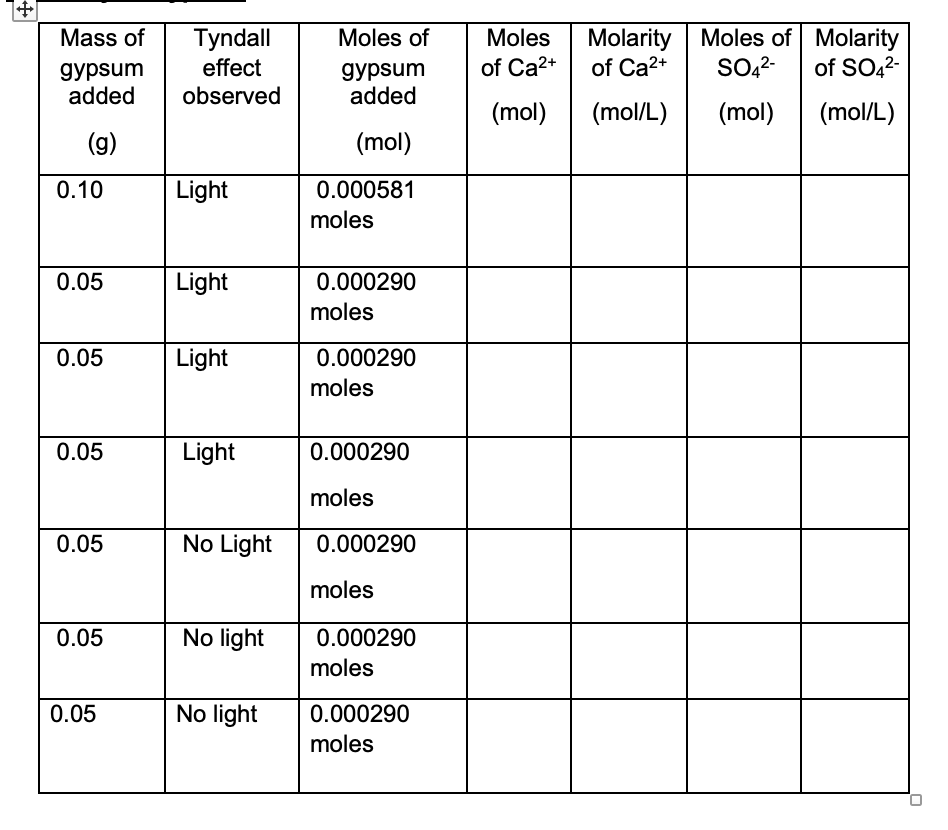
Solved Calculate The Moles And Molarity Of Ca 2 Calculate Chegg Com
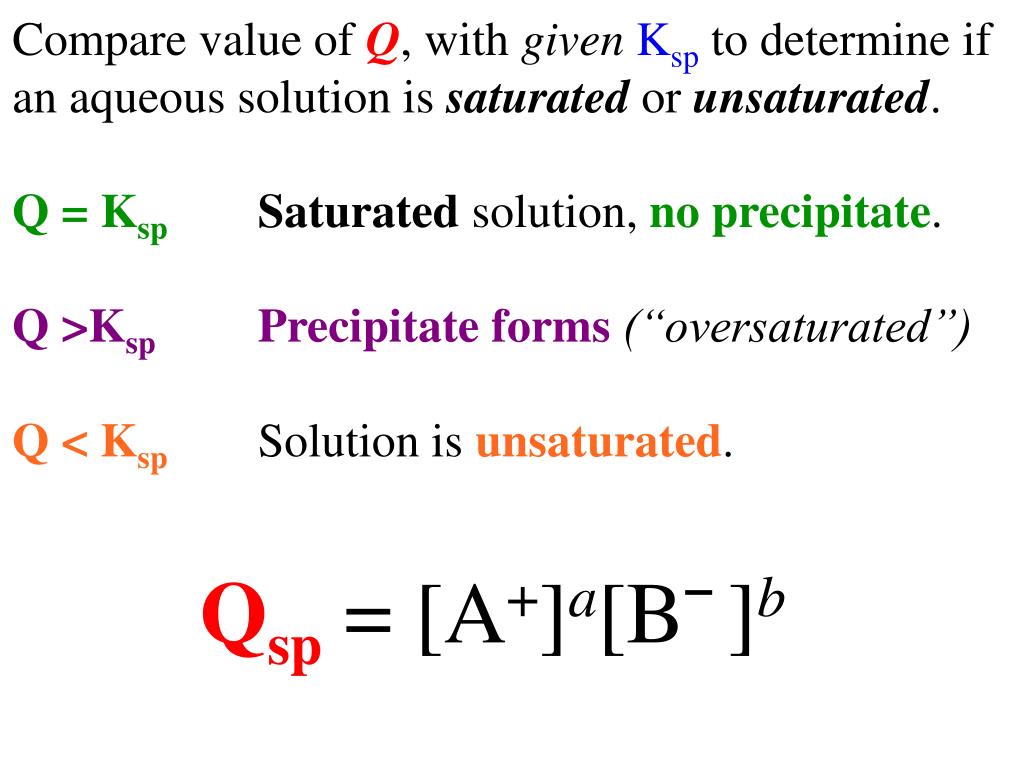
Web Ksp and Qsp.

. If Q Ksp then equilibrium has been reached and no MACROSCOPIC change will occur. Write the Q formula. This problem has been solved.
Web And Ksp M nXn And likewise we so define Q M nXn. You should always consider a horses QSP rating in the context of all the other horses in. Web So at this moment in time QSP is equal to 51 times 10 to the negative six.
Given the sample reaction. Web Multiply the result by the number of shares. The Relationship between Q.
Solubility Equilibria JFR Science 137K subscribers Subscribe 219 Share 14K views 5 years ago Mr. At 25 degrees Celsius the KSP value for lead two sulfate is equal to 63 times 10 to the negative. Next if you are buying items that cost more than 250000 that is their minimum limit today.
Web Step 1. Web Q Ksp. Web To find the Qsp of a reaction we have to calculate the product of the concentration of ions in the aqueous solution.
Web The QSP rating can be a very powerful tool for your personal pace handicapping analysis. Youll get a detailed solution from a subject matter expert that. Would the Qsp.
Web To get your answer find the number of moles of CaCO3 formed from the given data and the equation for the reaction. You may assume temperature is held. Web Net ionic equation 2AgCl - 2Ag Cl2 Ag before 00100 after 00050 Cl- before 00500 after 00250 My question was about the Qsp.
AB A - aq B aq The Qsp will be equal. It has been as low as 1000 you can submit your list at the Pro. Web Precipitation calculation using Qsp and Ksp 1456 views Mar 20 2021 7 Dislike Share Save CHEM1142 254 subscribers We look at how to calculate Qsp and.
Use the Ksp expression to find the number of moles of Ca2. The solution is supersaturated and ionic solid will precipitate. Q c C O 2 H 2 C O H 2 O Step 2.
The solution is supersaturated and ionic solid will precipitate. Web Calculate the Qsp or Ksp as indicated and determine whether a precipitate will form when each of the following mixtures is prepared. Web Calculate the Qsp for each amount of gypsum added.
Web At 25 degrees Celsius the KSP value for lead two sulfate is equal to 63 times 10 to the negative. 100 mL of Solution. 25 - 2125 x 100 375 Even if your employer didnt include the bargain amount in Box 1 of Form W-2 you report this.
The solution is saturated and at equilibrium. Key explains how the solubility product constant. Begin align Q_c dfrac 20 20.

Solved 1 Calculate The Solubility Of Ca3 Po4 2 Ksp 1 3x10 32 In A 0 035m Ca No3 2 Solution A 8 7x10 15 Mol Lb 1 7 X10 14 Mol Lc 3 0 X10 16 Mol Ld 7 6x10 29 Mol L2 You Have 75 0 Ml Of 0 14m Ha After

Calculate Qsp To Inr Live Today Qsp Inr Coinmarketcap

Solubility And Complex Ion Equilibria Ppt Download

The Solubility Product Principle 2 Silver Chloride Agcl Is Rather Insoluble In Water Careful Experiments Show That If Solid Agcl Is Placed In Pure Ppt Download

Ksp An Overview With Objectives And Examples Ppt Video Online Download
Ppt Solubility Equilibria Powerpoint Presentation Free Download Id 4869942

Week 10 12 Precipitation Calculation Using Qsp And Ksp Youtube

Universal Adapter Ring Formula

If Qsp Ksp Shift To Products No Precipitate Forms Ppt Download

The Solubility Product Principle 2 Silver Chloride Agcl Is Rather Insoluble In Water Careful Experiments Show That If Solid Agcl Is Placed In Pure Ppt Download

Solubility Constants Ksp Ppt Download

Solved When 5 0 Ml Of 0 012 M Pb No3 2 Are Mixed With 5 0 Ml Chegg Com
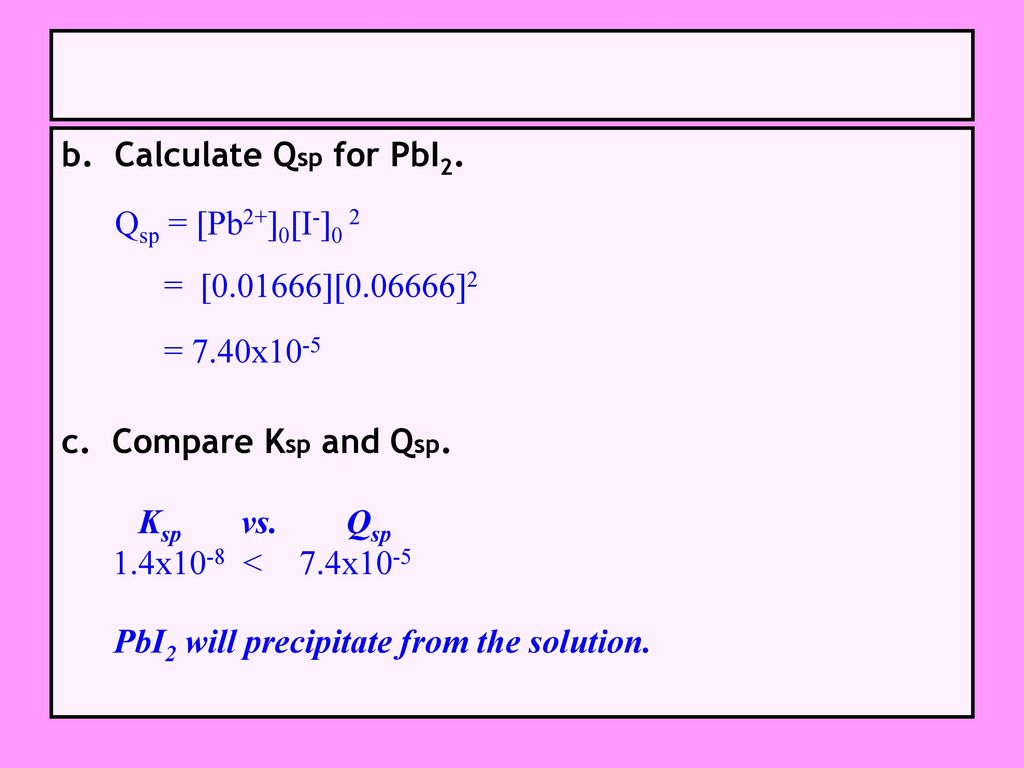
Ch 15 Part Iii Solubility Ksp And Precipitation Ppt Download
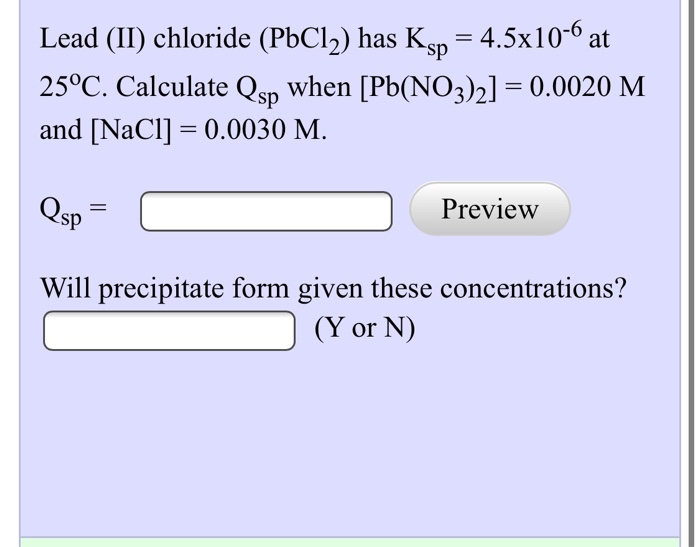
Solved Lead Ii Chloride Pbcl2 Has Ksp 4 5x10 6 At Chegg Com

Solved Calculate Qsp For Calcium Fluoride Ksp 3 9 10 11 Chegg Com

Complex Ions Precipitation And Molar Solubility Youtube
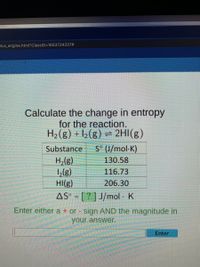
Answered Calculate The Change In Entropy For The Bartleby